Essure MedTruth Prescription Drug amp Medical Device
WEB FDA Publishes New Essure Labeling and Safety Checklist Ashley Lombardo 183 November 16 2016 The FDA issued an Essure safety checklist to help physicians and
FDA Takes Additional Action To Better Understand Safety Of Essure , WEB The FDA also intends to require changes to product labeling including a boxed warning and a Patient Decision Checklist to help to ensure women receive and understand

Information For Patients And Health Care Providers Essure
WEB Oct 30 2023 nbsp 0183 32 Required Patient Doctor Discussion Checklist for Essure In 2016 the FDA approved important labeling changes for Essure to provide important information about
Essure Problems Advocates Address FDA With Letter Signed By, WEB June 2 2017 By Ashley Lombardo Essure Problems an advocacy group for women injured by a popular birth control device addressed a letter to the Food and Drug
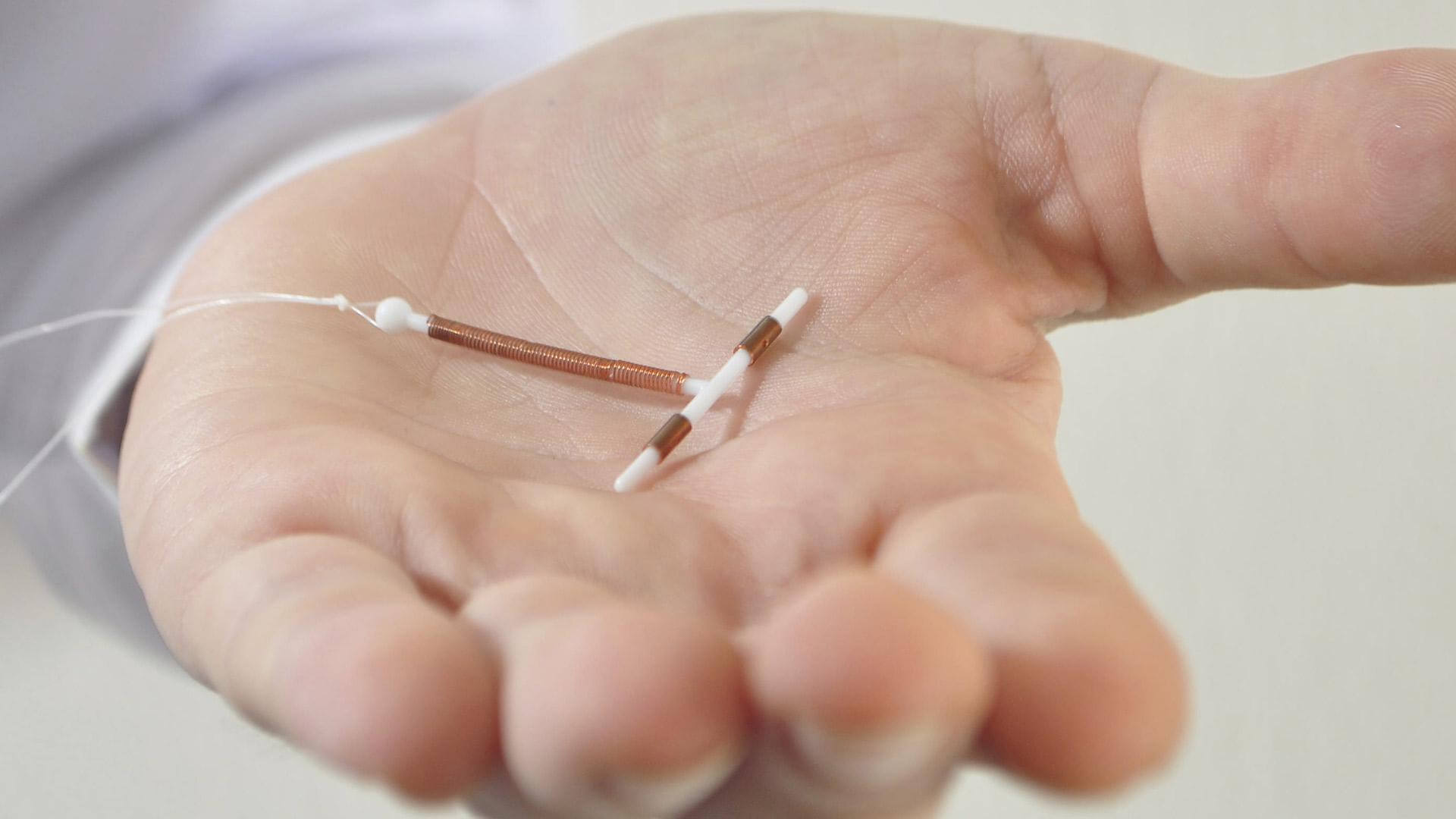
Research Findings MedTruth Prescription Drug amp Medical
Research Findings MedTruth Prescription Drug amp Medical , WEB Jun 11 2018 nbsp 0183 32 FDA Publishes New Essure Labeling and Safety Checklist Ashley Lombardo 183 November 16 2016 The FDA issued an Essure safety checklist to help

Safety Of Essure Contraceptive Device Gets Another Look Shots
FDA Restricts Sale And Distribution Of Essure To Protect Women And
FDA Restricts Sale And Distribution Of Essure To Protect Women And WEB The new Essure labeling which will now be legally required when this product is offered to a patient restricts the sale and distribution of the device to only health care providers and

FDA Reviewing Safety Of Essure Birth Control Implant
WEB Nov 23 2016 nbsp 0183 32 The Patient Decision Checklist provides information about the device s use safety and effectiveness outcomes Officials with Bayer revised the patient Essure Receives New Boxed Warning And Patient Decision Checklist. WEB November 18 2016 Labeling Changes to Bayer s Essure Device Include Boxed Warning By MedTech Intelligence Staff Approved by FDA the changes include a warning and a WEB The FDA has taken a number of actions over the last several years to ensure the safety of the Essure device Beginning in 2015 the FDA convened a public meeting to learn more

Another Fda Publishes New Essure Labeling And Safety Checklist Medtruth you can download
You can find and download another posts related to Fda Publishes New Essure Labeling And Safety Checklist Medtruth by clicking link below
- Urban Harvest Resources Good To Grow
- After Years Of Complaints Bayer Forced To Stop Selling Essure
- New Stronger FDA Warning For Essure Birth Control Implant Orange
- Essure Patient Information Will Now Need Risks Checklist Signed By
- Solved Suppose That The Seitan Industry Is Initially Operating In
Thankyou for visiting and read this post about Fda Publishes New Essure Labeling And Safety Checklist Medtruth