What Is The Lewis Structure Of Ozone O 3 Toppr
What is the shape of ozone molecule Write Lewis dot structure s for ozone molecule and explain why O O distances in ozone are equal
What Is The Lewis Structure SO 2 Toppr, The electron geometry of S O 2 is formed in the shape of a trigonal planner The three pairs of bonding electrons arranged in the plane at the angle of 120 degree As the one pair remained
![]()
Draw The Structure Of O 3 And NO 2 Toppr
Draw Lewis structure for the following molecules i SF 6 ii C2H 4 iii N O2 View Solution
What Is The Molecular Geometry Of CCl 4 Draw Its VSEPR And , The Lewis structure shows that there are four electron regions about the central carbon atom The VSEPR model states that the electron regions around an atom spread out to make each region

SO
SO , Lewis SO 1 5
Fluoroform Lewis Structure
Write The Lewis Structure Of The Nitrite Ion NO 2 Toppr
Write The Lewis Structure Of The Nitrite Ion NO 2 Toppr Lewis structures also known as Lewis dot diagrams Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures LEDS are diagrams that show the

Lewis Structure For Helium
In the Lewis structure the outermost orbit electrons of each atom is shown The Lewis structure for CO2 3 is shown in the figure Draw The Lewis Structure CO 3 2 Toppr. Resonance structures are the multiple Lewis structures of similar energy the position of nuclei bonding and the non bonding pair of electrons that can accurately describe a molecule N O C l is a strong electrophile and readily undergoes an addition reaction with alkenes Complete the diagram to show the mechanism of the electrophilic addition reaction of N O C l with

Another Lewis Structure you can download
You can find and download another posts related to Lewis Structure by clicking link below
- Lewis Structure Of SO2
- Lewis Structure For Hydrogen Vrogue co
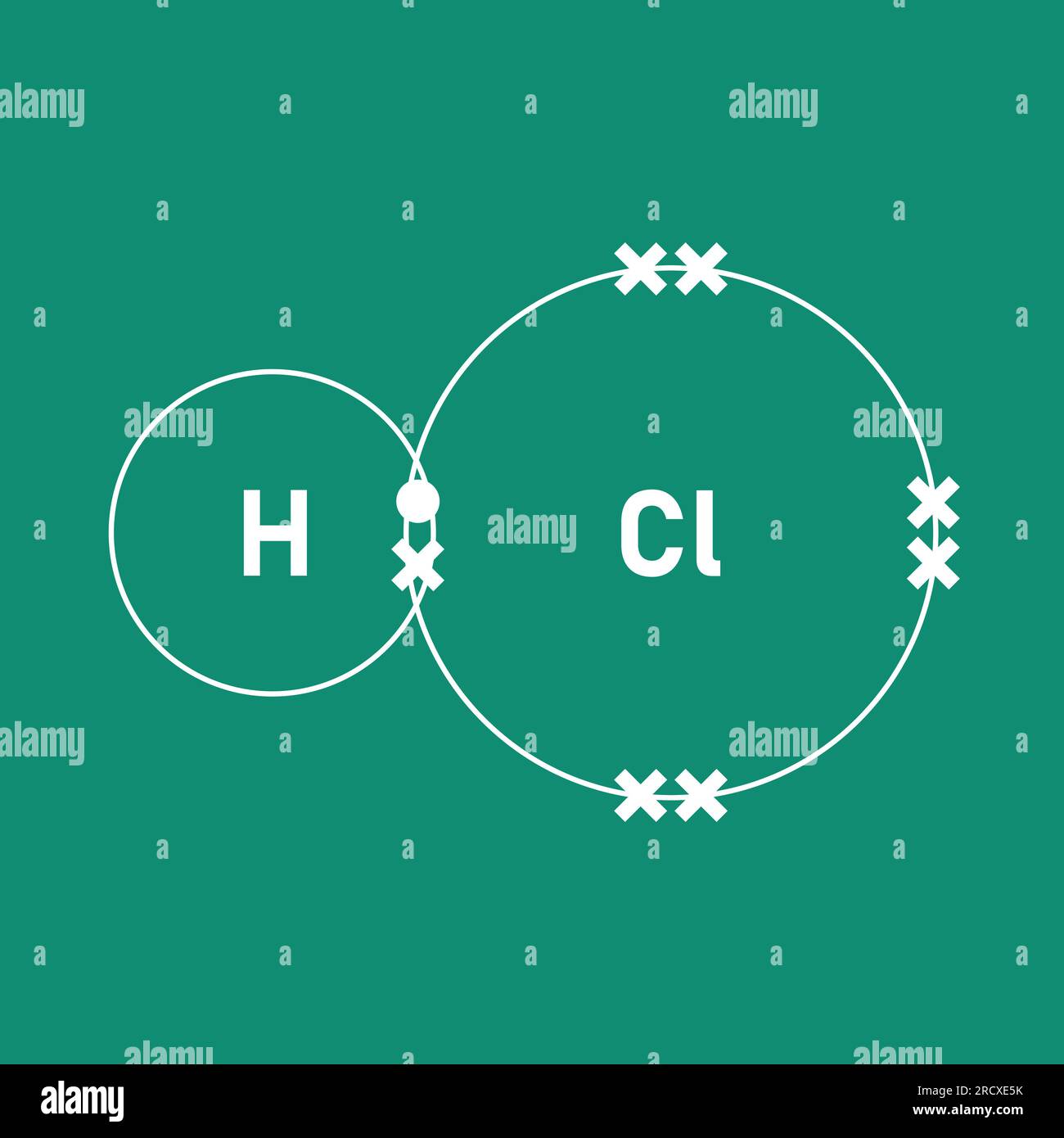
- Lewis Structure Of HCl How To Draw Molecular Geometry Hybridization
- AlI3 Lewis Structure In 5 Steps With Images
- SF3 Lewis Structure In 5 Steps With Images
Thankyou for visiting and read this post about Lewis Structure