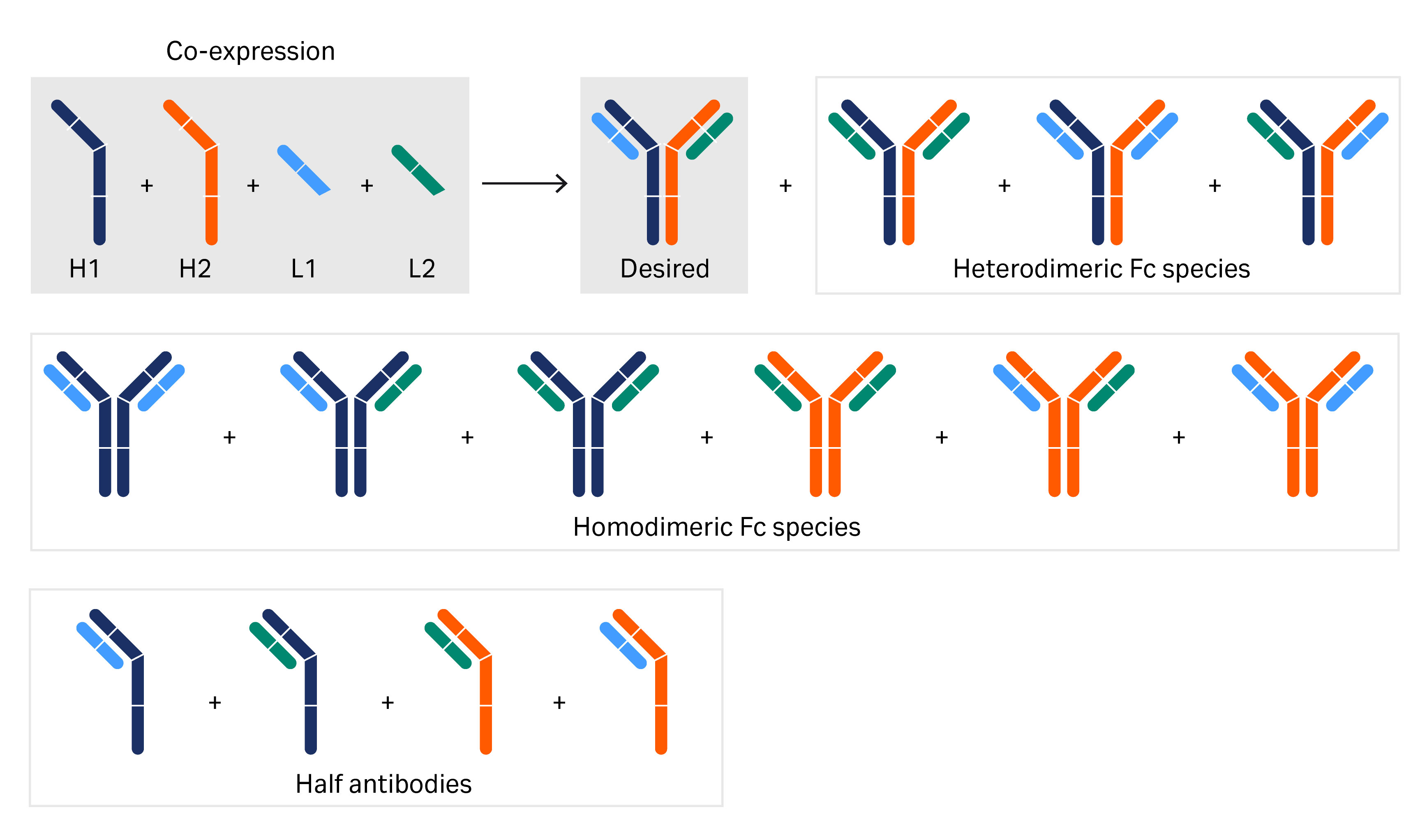
Methods To Produce Monoclonal Antibodies For The Prevention
WEB May 26 2022 nbsp 0183 32 An undeniable advantage of methods based on B cell screening is the direct functional analysis of antibodies obtained from natural antibody producing cells which reduces a number of risks associated with the use of other approaches for example changes in the structure of antibody molecules or the loss of some antibody variants
Production Of Monoclonal Antibodies For Therapeutic Purposes , WEB Jul 1 2023 nbsp 0183 32 Monoclonal antibodies can recognize and target specific epitopes on an antigen Common techniques to produce mAbs are Hybridoma Technology and Phage Display mAbs are produced by homologous B cells resulting from a single parent cell clone Novel perspectives of mAbs are use of continuous operations and plant hosts

Cell Culture Processes For Monoclonal Antibody Production
WEB Apr 14 2010 nbsp 0183 32 We also summarize the current thinking on appropriate process development strategies and process advances that might affect process development Key words monoclonal antibody expression systems cell line engineering cell culture process development optimization scale up and technology transfer process advances
Production Processes For Monoclonal Antibodies IntechOpen, WEB Feb 8 2017 nbsp 0183 32 Abstract Antibodies are glycoprotein structures with immune activity They are able to identify or induce a neutralizing immune response when they identify foreign bodies such as bacteria viruses or tumor cells Immunoglobulins are produced and secreted by B lymphocytes in response to the presence of antigens
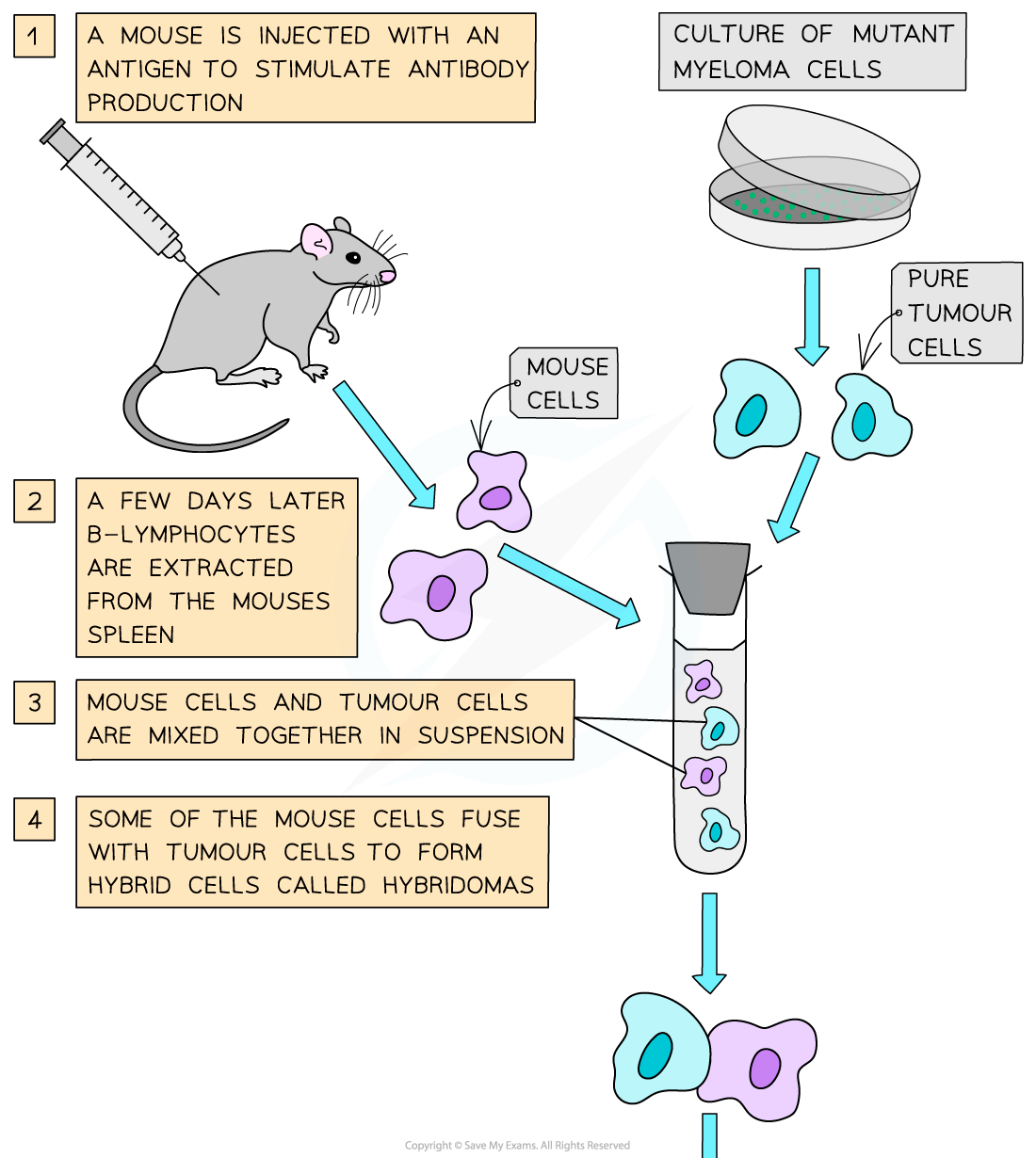
PDF Monoclonal Antibodies Methods And Protocols
PDF Monoclonal Antibodies Methods And Protocols, WEB Jan 1 2014 nbsp 0183 32 Described herein are methods for the successful screening of monoclonal antibodies mAbs of the desired specificities via high throughput HTP homogeneous assay and flow cytometry

B
Guidelines For The Production And Quality Control Of Monoclonal
Guidelines For The Production And Quality Control Of Monoclonal WEB Jun 7 2022 nbsp 0183 32 Guidelines for the production and quality control of monoclonal antibodies and related products intended for medicinal use Replacement of Annex 3 of WHO Technical Report Series No 822 Adopted by the Seventy fifth meeting of the World Health Organization Expert Committee on Biological Standardization 4 8 April 2022 This is
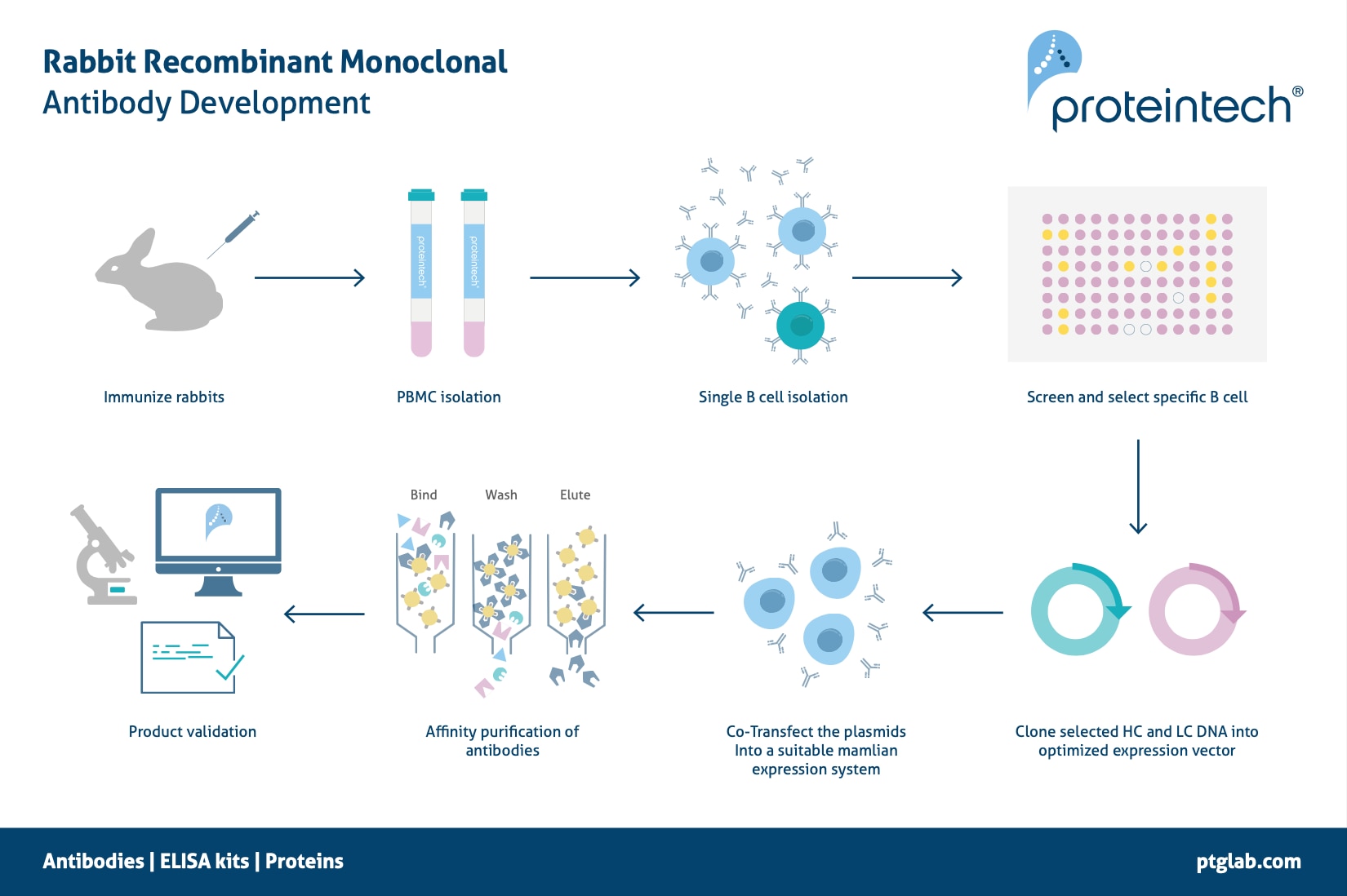
Purifying Bispecific Antibodies In A Single Step Cytiva
WEB Some manufacturers have gained considerable experience in the production of monoclonal antibodies and have developed a production strategy based on similar manufacturing processes i e using a pre defined host cell cell culture and purification process This approach is often referred to as platform manufacturing Guideline On Development Production Characterisation And . WEB Apr 21 2020 nbsp 0183 32 Developing therapeutic monoclonal antibodies at pandemic pace Brian Kelley Nature Biotechnology 38 540 545 2020 Cite this article 62k Accesses 105 Citations 241 Altmetric Metrics WEB Introduction Monoclonal antibodies mAb are important reagents used in biomedical research in diagnosis of diseases and in treatment of such diseases as infections and cancer These antibodies are produced by cell lines or clones obtained from animals that have been immunized with the substance that is the subject of study
Another Monoclonal Antibodies From Screening To Production you can download
You can find and download another posts related to Monoclonal Antibodies From Screening To Production by clicking link below
- Antibody Essentials Part 4 Polyclonal Vs Monoclonal Antibodies
- Refinar Emp rico Troca Monoclonal Antibody Production Process Flow
- Monoclonal Antibodies Mabs Production By Hybridoma Technology
- Production Of Anti CD20 Monoclonal Antibodies Procedure Of Cell Based
- 20 1 Aplicaciones Pr cticas De Anticuerpos Monoclonales Y Policlonales
Thankyou for visiting and read this post about Monoclonal Antibodies From Screening To Production