What Is Rusting Toppr
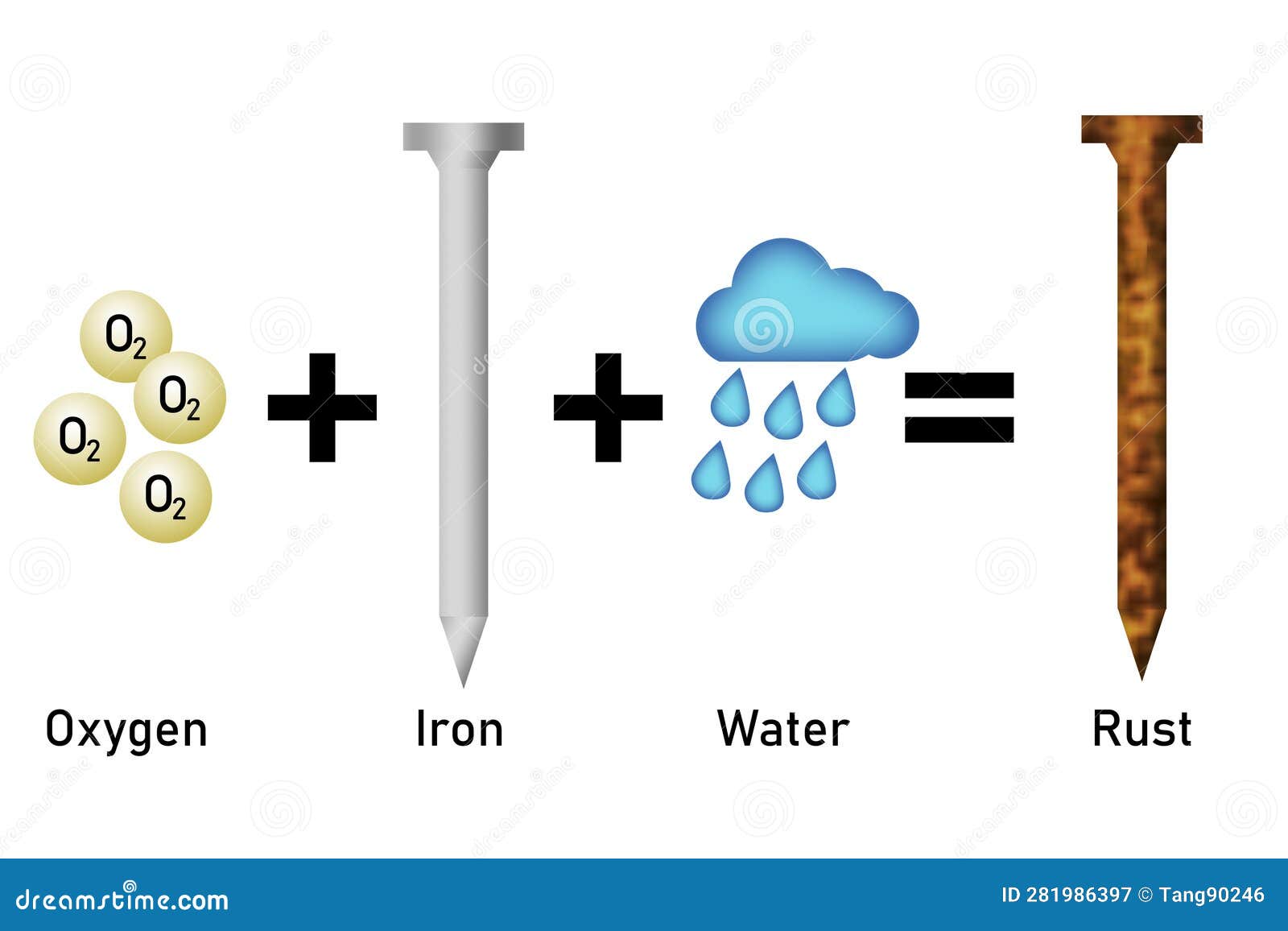
Iron objects often acquire a thin film of brownish substance on their outer coating This substance is rust and the process of its formation is rusting Chemically when iron comes in contact with oxygen and water a reaction takes place to form iron oxide rust
What Is Rust BYJU S, What is Rust Iron in the presence of moisture combines with the oxygen from the air to form a brown coloured chemical substance called rust iron oxide There is no physical process to get back iron from the rust iron oxide Rusting is a costly nuisance Methods of slowing down the process can save a lot of money Many metals become corroded by exposure to the air The

What Is Rusting Give The Equation The Formation Of Rust Toppr
Rusting is the slow oxidation of iron by atmospheric oxygen in the presence of water
What Is Rusting BYJU S, Rusting is the common term for corrosion of iron and its alloys such as steel Other metals undergo equivalent corrosion but the resulting oxides are not commonly called rust Given sufficient time oxygen and water any iron mass eventually converts entirely to rust and disintegrates Rust is a general term for a series of iron oxides usually red oxides formed by

Difference Between Rust And Corrosion BYJU S
Difference Between Rust And Corrosion BYJU S, In simple terms corrosion is a type of oxidation whereas rusting is a part of corrosion The main difference though between corrosion and rust is that corrosion occurs as a result of the chemical influence and it affects a lot of materials whereas rusting is only accelerated by certain chemicals and usually affects iron substances
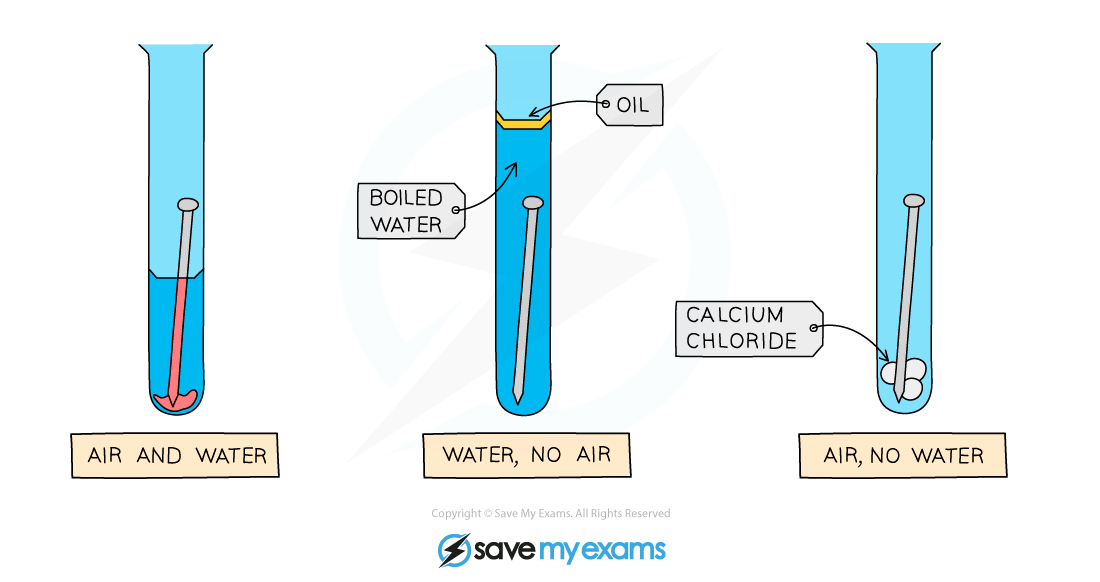
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 2 4 4 Rusting Of Iron
Corrosion Chemistry Questions With Solutions BYJU S
Corrosion Chemistry Questions With Solutions BYJU S Corrosion Chemistry Questions with Solutions Q1 Which of the following is an example of corrosion a Rusting of iron b Tarnishing of silver c Liquefaction of ammonia d Rusting of iron and tarnishing of silver Answer d Rusting of iron and tarnishing of silver Explanation Corrosion caused by the oxidation process includes rusting of iron and tarnishing of silver Q2 Metals do

Rusting Process Chemical Reaction Vector Illustration CartoonDealer
The reaction of the rusting of iron involves an increase in the oxidation state of iron accompanied by a loss of electrons Rust is mostly made up of two different oxides of iron that vary in the oxidation state of the iron atom These oxides are 1 Iron II oxide or ferrous oxide Rusting Of Iron Is An Example Of Toppr. Rusting of iron takes place in the presence of moisture and air So the presence of oxygen and water vapour in air are two necessary conditions for rusting of iron Strong electrolytes such as sodium chloride are present in saline water The ions produced from N a C l help in the reduction of oxygen to form water Hence the rusting of iron is faster in saline water than in pure water
Another Rusting Of Iron Nail Experiment Diagram Stock Illustration you can download
You can find and download another posts related to Rusting Of Iron Nail Experiment Diagram Stock Illustration by clicking link below
- Write Short Essay On Prevention Of Rusting Brainly in
- 21 333 Rusted Iron Nail Stock Photos Images Photography Shutterstock
- Learning Task No 5 Investigating The Rusting Of Iron You Will Need
- Learning Task No 5 Investigating The Rusting Of IronYou Will Need The
- Premium Vector Iron Nail Clipart
Thankyou for visiting and read this post about Rusting Of Iron Nail Experiment Diagram Stock Illustration